BBA - Molecular Basis of Disease (2022)
Julian Wolf, Anja Schlecht, Dennis-Dominik Rosmus, Stefaniya Boneva, Hansjürgen Agostini, Günther Schlunck, Peter Wieghofer and Clemens Lange
Number of citations (crossref.org): Loading....

Background: Visual outcome of patients with neovascular
age-related macular degeneration has significantly improved during the last
years following the introduction of anti-vascular endothelial growth factor
(VEGF) therapy. However, about one third of patients show persistent exudation
and decreasing visual acuity despite recurrent anti-VEGF treatment, which
implies a role of other, still unknown proangiogenic mediators.
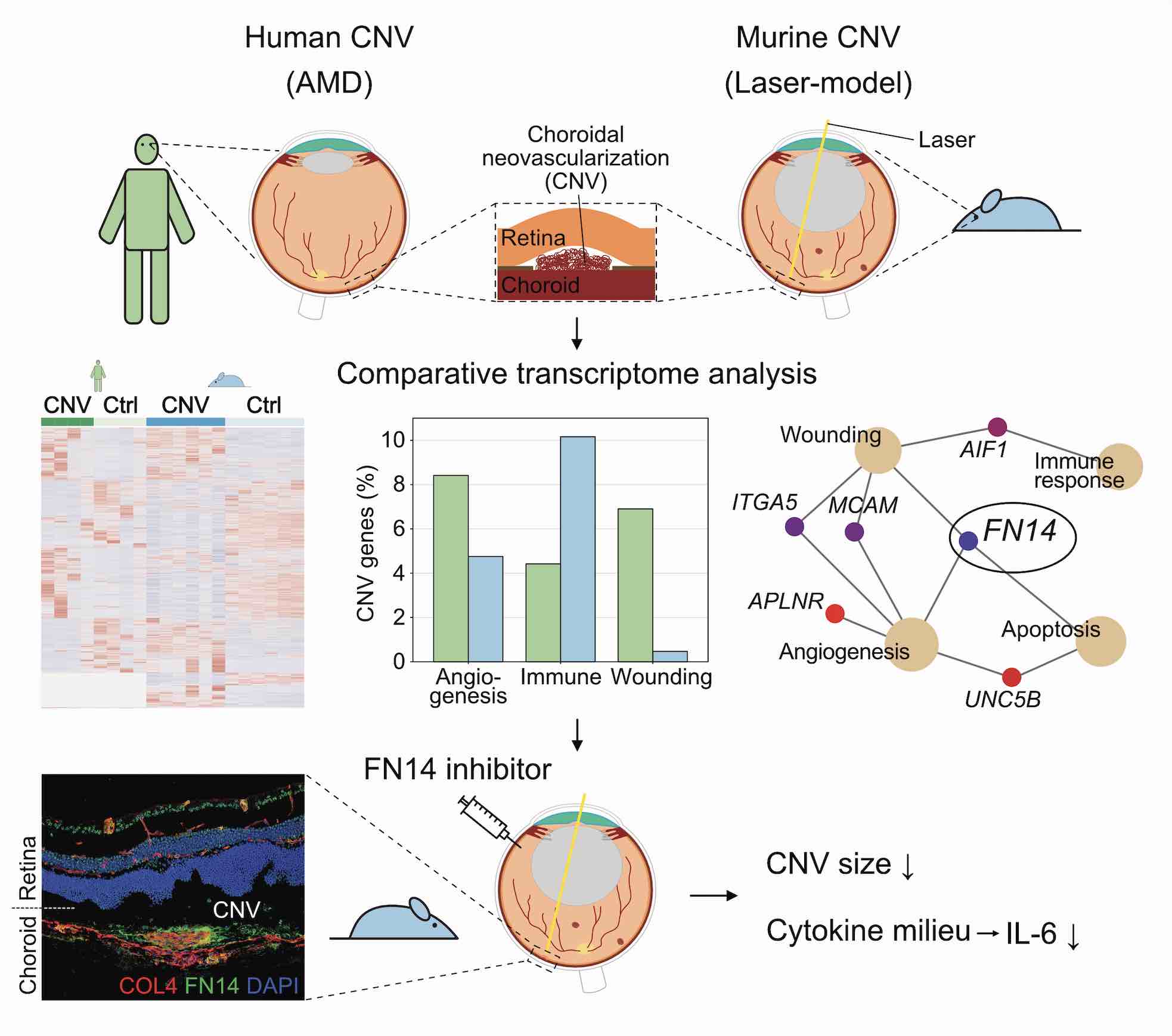
Methods: The present study applied transcriptional profiling
of human and mouse (C57BL/6J wildtype) choroidal neovascularization (CNV)
membranes each with reference to healthy control tissue to identify yet
unrecognized mediators of CNV formation. Key factors were further investigated
by immunohistochemistry as well as by intravitreal inhibition experiments and
multiplex protein assays in the laser-induced CNV mouse model.
Findings: Transcriptional profiles of CNV membranes were
characterized by enhanced activation of blood vessel development, cytoskeletal
organization, and cytokine production, with angiogenesis and wound healing
processes predominating in humans and activation of immune processes in mice.
Besides several species-specific factors, 95 phylogenetically conserved
CNV-associated genes were detected, among which fibroblast growth factor
inducible-14 (FN14), a member of the tumor necrosis factor (TNF) receptor
family, was identified as a key player of CNV formation. Blocking the
pathway by intravitreal injection of a FN14 decoy receptor modulated the
cytokine profile - most notably IL-6 - and led to a significant reduction
of CNV size in vivo.
Interpretation: This study characterizes the transcriptome
of human and mouse CNV membranes in an unprejudiced manner and identifies
FN14 as a phylogenetically conserved mediator of CNV formation and a
promising new therapeutic target for neovascular AMD.