Frontiers in Cell and Developmental Biology (2021)
Schlecht A, Zhang P, Wolf J, Thien A, Rosmus D, Boneva S, Schlunck G, Lange C * and Wieghofer P *
* contributed equally
Number of citations (crossref.org): Loading....

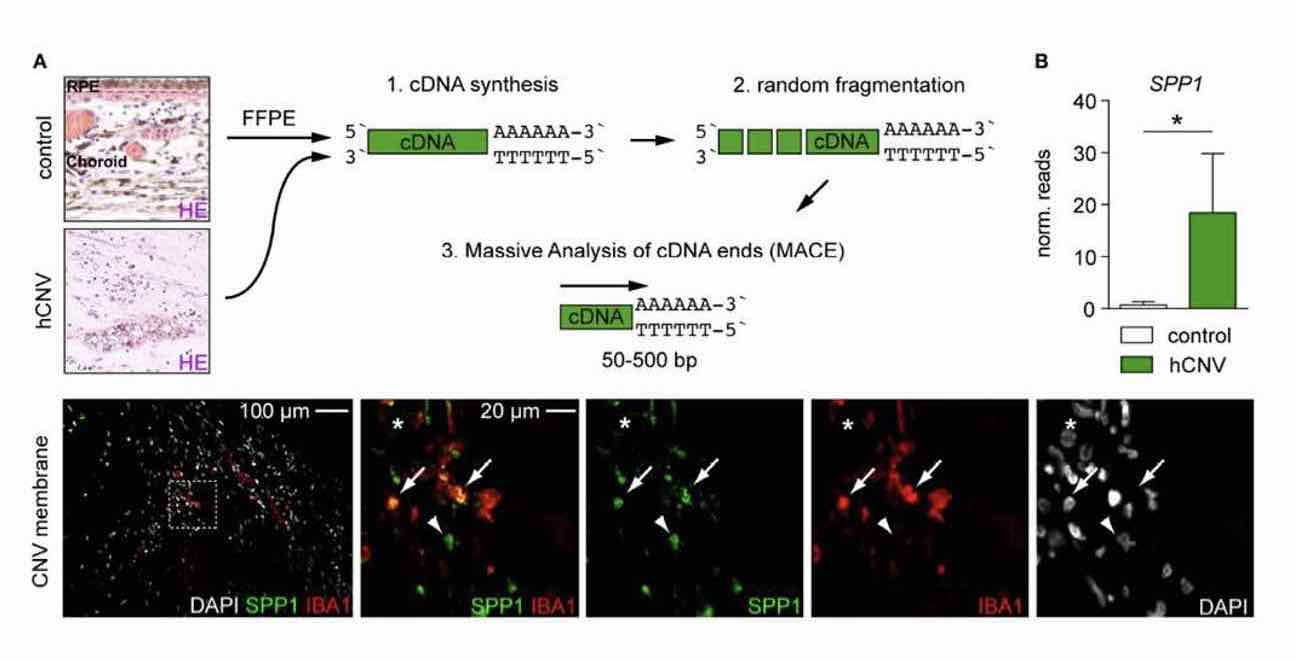
Age-related macular degeneration (AMD) represents the most common cause of blindness in the elderly in the Western world. An impairment of the outer blood-retina barrier and a localized inflammatory microenvironment cause sprouting of choroidal neovascular membranes (CNV) in neovascular AMD that are in intimate contact with surrounding myeloid cells, such as retinal microglia, and ultimately lead to visual impairment. The discovery of novel target molecules to interfere with angiogenesis and inflammation is vital for future treatment approaches in AMD patients. To explore the transcriptional profile and the function of retinal microglia at sites of CNV, we performed a comprehensive RNA-seq analysis of retinal microglia in the mouse model of laser-induced choroidal neovascularization (mCNV). Here, we identified the angiogenic factor Osteopontin (Opn), also known as “secreted phosphoprotein 1” (Spp1), as one of the most highly expressed genes in retinal microglia in the course of CNV formation. We confirmed the presence of SPP1 at the lesion site in recruited retinal microglia in Cx3cr1CreER:Rosa26-tdTomato reporter mice by confocal microscopy and in whole retinal tissue lysates by ELISA highlighting a massive local production of SPP1. Inhibition of SPP1 by intravitreal injection of an anti-SPP1 antibody significantly increased the lesion size compared to IgG-treated control eyes. In line with our results in rodents, we found an increased SPP1 mRNA expression in surgically extracted human choroidal neovascular (hCNV) membranes by the quantitative RNA-seq approach of massive analysis of cDNA ends (MACE). Numerous IBA1+SPP1+ myeloid cells were detected in human CNV membranes. Taken together, these results highlight the importance of SPP1 in the formation of CNV and potentially offer new opportunities for therapeutic intervention by modulating the SPP1 pathway.